الأكسجين المذاب والفقاعات النانوية
مقدمة

يشير الأكسجين المذاب، أو DO، إلى مستوى الأكسجين الحر غير المركب الموجود في الماء أو السوائل الأخرى. وتعد قيمة DO معلمة أساسية لجودة المياه والعملية التي تستخدم فيها المياه. والفقاعة متناهية الصغر، والمعروفة أيضًا باسم الفقاعة النانوية، ليست أكسجينًا مذابًا؛ فالفقاعة هي تجويف غازي في الماء أو سائل آخر.
تقليدياً، التهوية هي تقنية لزيادة قيمة DO في الماء. والآن، مع تقنية الفقاعات النانوية، لدينا مستويين لزيادة الأكسجين في الماء: المستوى الأول هو الأكسجين المذاب، والمستوى الثاني هو عبر الفقاعات أو التجاويف الغازية في الماء. ولهذا السبب، نشير أيضًا إلى توليد الفقاعات النانوية كتقنية تهوية معززة.
تؤثر العوامل التالية على مستوى الأكسجين المذاب:
- درجة حرارة الماء
- ملوحة الماء
- ارتفاع التشغيل (الضغط الجوي)
- تتأثر البيولوجيا في الماء بتنفس الأسماك والنباتات
العلاقة بين درجة حرارة الماء و DO عكسية: يمكن للمياه الباردة أن تحتفظ بكمية أكبر من DO من المياه الدافئة. تضغط مولدات الفقاعات متناهية الصغر في هذا الموقع على الغازات والسوائل؛ وبالتالي، يمكن أن تزيد من تشبع الماء. في الطبيعة، في الظروف العادية، يكون مستوى التشبع 100٪ هو الحد الأقصى.
يحتوي الهواء على 20.95% من الأكسجين. وعند الضغط البارومتري القياسي (760 ملم زئبق)، يبلغ ضغط أو "توتر" الأكسجين في الهواء 159 ملم زئبق (760 × 0.2095). يدفع ضغط الأكسجين في الهواء الأكسجين إلى الماء حتى يصبح ضغط الأكسجين في الماء مساوياً لضغط الأكسجين في الغلاف الجوي. عندما يتساوى ضغط الأكسجين في الماء والغلاف الجوي، تتوقف الحركة الصافية لجزيئات الأكسجين من الغلاف الجوي إلى الماء. عندئذٍ يكون الماء في حالة توازن أو تشبع بالأكسجين المذاب (DO)، عندما يكون ضغط الأكسجين في الماء مساوياً لضغط الأكسجين في الغلاف الجوي.
جزء في المليون مقابل ملغم/لتر
كثيراً ما نتساءل عن الفرق بين DO جزء في المليون و DO ملغم/لتر. للوهلة الأولى، يبدو للوهلة الأولى أنهما شكلان مختلفان تمامًا للقياس. كلاهما نسب، ولمعرفة كيفية توافقهما مع بعضهما البعض، من الأسهل البدء بالجزء في المليون، أو جزء في المليون. على سبيل المثال، لنفترض أنك تحاول تحديد ملوحة مياه البحر، وتحصل على قراءة 36,000 جزء في المليون؛ وهذا يعني أنه لكل مليون جزء من الماء، هناك 36,000 جزء من الملح.
ما هي الأجزاء؟ يمكن قياس الأجزاء بأي وحدة: لتر أو دلو أو قطرة ماء (مثل عصير البرتقال أو البنزين). حجم العينة غير ذي صلة. المهم هو نسبة الأجزاء المختبرة (الملح) إلى العدد الإجمالي للأجزاء (ماء البحر). من السهل فهم جزء في المليون، ولكن ماذا عن ملغم/لتر؟
يزن لتر الماء (وهو مقياس متري للحجم أو السعة) 1 كيلوغرام. أي 1,000 جرام. والآن فكر في الملليغرام. إنه يساوي 1/1000 من الجرام، مما يجعله يساوي 1/1,000,000 من الكيلوجرام. وبعبارة أخرى، يزن لتر من الماء 1,000,000,000 ملليغرام. مليون ملليغرام ... أترى إلى أين يقودنا هذا؟ بالنسبة لأغراضنا، فإن 36,000 ملليغرام في اللتر الواحد يعادل 36,000 جزء من المليون.* كلا القياسين يخبرنا بعدد الأجزاء (الملليغرامات) الموجودة في كل مليون جزء (لتر).
في الواقع، لكي يكون هذان القياسان متساويين تمامًا، يجب أن يتم أخذهما بماء نقي عند درجة حرارة وضغط قياسيين. تتضمن معظم أدوات الاختبار ميزة التعويض التلقائي لدرجة الحرارة (ATC)، والتي تصحح هذا الاختلاف.
جدول قيم DO
قيم الأكسجين المذاب ونقطة التشبع وقيم التشبع الزائد
| درجة الحرارة | DO (ملغم/لتر) | DO (ملغم/لتر) | DO (ملغم/لتر) | DO (ملغم/لتر) | DO (ملغم/لتر) |
|---|---|---|---|---|---|
| (درجة مئوية) | 100% | 200% | 300% | 400% | 500% |
| 0 | 14.6 | 29.2 | 43.8 | 58.4 | 73 |
| 1 | 14.19 | 28.38 | 42.57 | 56.76 | 70.95 |
| 2 | 13.81 | 27.62 | 41.43 | 55.24 | 69.05 |
| 3 | 13.44 | 26.88 | 40.32 | 53.76 | 67.2 |
| 4 | 13.09 | 26.18 | 39.27 | 52.36 | 65.45 |
| 5 | 12.75 | 25.5 | 38.25 | 51 | 63.75 |
| 6 | 12.43 | 24.86 | 37.29 | 49.72 | 62.15 |
| 7 | 12.12 | 24.24 | 36.36 | 48.48 | 60.6 |
| 8 | 11.83 | 23.66 | 35.49 | 47.32 | 59.15 |
| 9 | 11.55 | 23.1 | 34.65 | 46.2 | 57.75 |
| 10 | 11.27 | 22.54 | 33.81 | 45.08 | 56.35 |
| 11 | 11.01 | 22.02 | 33.03 | 44.04 | 55.05 |
| 12 | 10.76 | 21.52 | 32.28 | 43.04 | 53.8 |
| 13 | 10.52 | 21.04 | 31.56 | 42.08 | 52.6 |
| 14 | 10.29 | 20.58 | 30.87 | 41.16 | 51.45 |
| 15 | 10.07 | 20.14 | 30.21 | 40.28 | 50.35 |
| 16 | 9.85 | 19.7 | 29.55 | 39.4 | 49.25 |
| 17 | 9.65 | 19.3 | 28.95 | 38.6 | 48.25 |
| 18 | 9.45 | 18.9 | 28.35 | 37.8 | 47.25 |
| 19 | 9.26 | 18.52 | 27.78 | 37.04 | 46.3 |
| 20 | 9.07 | 18.14 | 27.21 | 36.28 | 45.35 |
| 21 | 8.9 | 17.8 | 26.7 | 35.6 | 44.5 |
| 22 | 8.72 | 17.44 | 26.16 | 34.88 | 43.6 |
| 23 | 8.56 | 17.12 | 25.68 | 34.24 | 42.8 |
| 24 | 8.4 | 16.8 | 25.2 | 33.6 | 42 |
| 25 | 8.24 | 16.48 | 24.72 | 32.96 | 41.2 |
| 26 | 8.09 | 16.18 | 24.27 | 32.36 | 40.45 |
| 27 | 7.95 | 15.9 | 23.85 | 31.8 | 39.75 |
| 28 | 7.81 | 15.62 | 23.43 | 31.24 | 39.05 |
| 29 | 7.67 | 15.34 | 23.01 | 30.68 | 38.35 |
| 30 | 7.54 | 15.08 | 22.62 | 30.16 | 37.7 |
| 31 | 7.41 | 14.82 | 22.23 | 29.64 | 37.05 |
| 32 | 7.28 | 14.56 | 21.84 | 29.12 | 36.4 |
| 33 | 7.16 | 14.32 | 21.48 | 28.64 | 35.8 |
| 34 | 7.05 | 14.1 | 21.15 | 28.2 | 35.25 |
| 35 | 6.93 | 13.86 | 20.79 | 27.72 | 34.65 |
| 36 | 6.82 | 13.64 | 20.46 | 27.28 | 34.1 |
| 37 | 6.71 | 13.42 | 20.13 | 26.84 | 33.55 |
| 38 | 6.61 | 13.22 | 19.83 | 26.44 | 33.05 |
| 39 | 6.51 | 13.02 | 19.53 | 26.04 | 32.55 |
| 40 | 6.41 | 12.82 | 19.23 | 25.64 | 32.05 |
| 41 | 6.31 | 12.62 | 18.93 | 25.24 | 31.55 |
| 42 | 6.22 | 12.44 | 18.66 | 24.88 | 31.1 |
| 43 | 6.13 | 12.26 | 18.39 | 24.52 | 30.65 |
| 44 | 6.04 | 12.08 | 18.12 | 24.16 | 30.2 |
| 45 | 5.95 | 11.9 | 17.85 | 23.8 | 29.75 |
ذوبانية الأكسجين في الماء
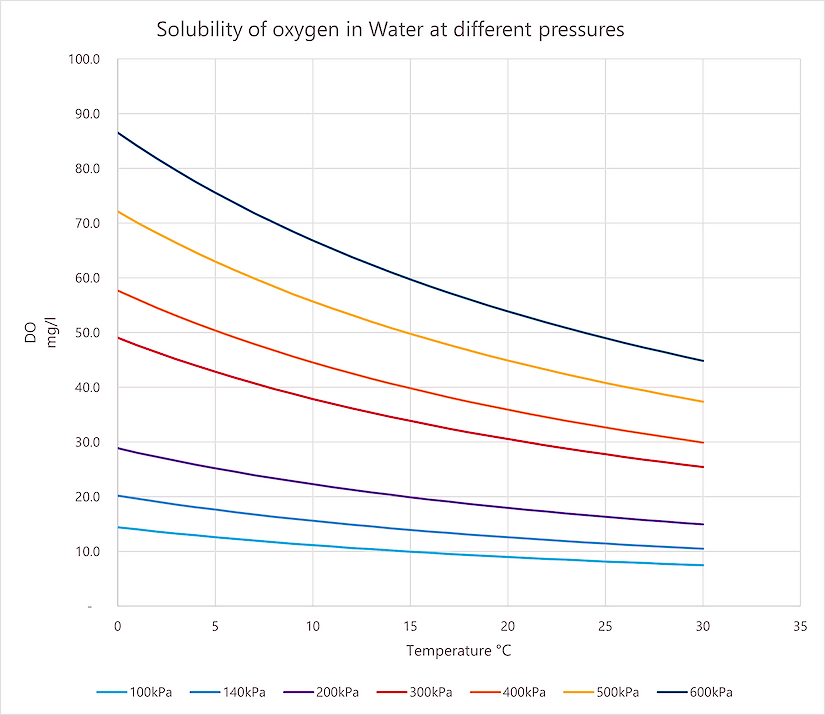
رسم بياني لقابلية ذوبان الأكسجين في الماء عند ضغوط مختلفة. عند اختيار مكثف الأكسجين، تأكد من مطابقته لإعدادات الضغط المطلوبة.
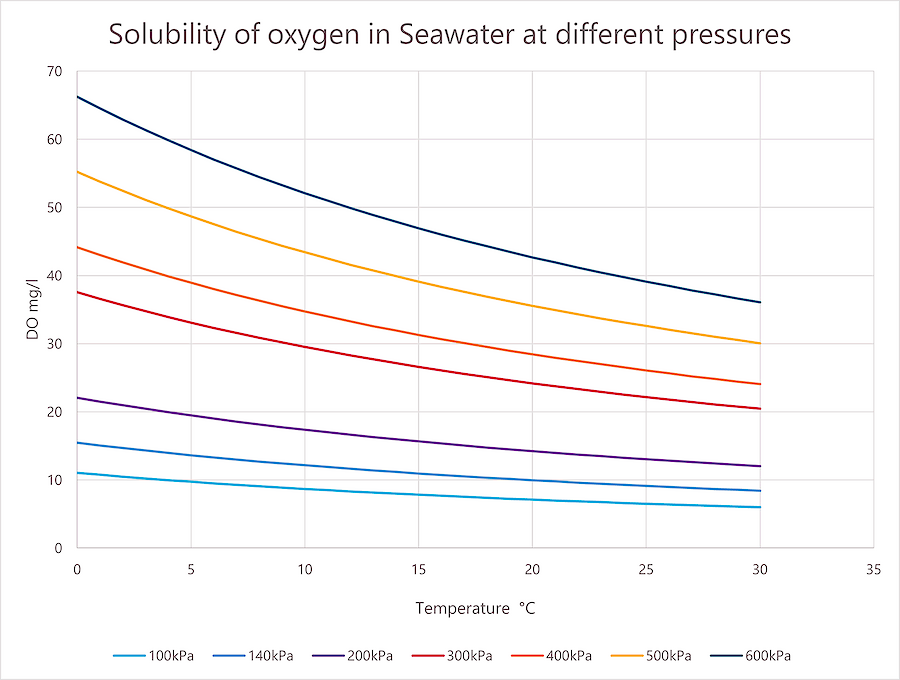
رسم بياني لذوبان الأكسجين في ماء البحر عند ضغوط مختلفة. عند الحاجة إلى رسم بياني بملوحة مختلفة، اتصل بنا لإجراء عملية حسابية.
التوزيع التقريبي للغازات الذائبة في الماء وفي الهواء
| الغازات | النسبة المئوية في الهواء | النسبة المئوية التقريبية لإجمالي الغازات الذائبة في الماء | التركيز (ملغم/لتر) |
|---|---|---|---|
| النيتروجين (N₂) | 78.1% | ~62-65% | ~13-14 ملغم/لتر |
| الأكسجين (O₂) | 20.9% | ~30-34% | ~8-9 ملغم/لتر |
| الأرجون (Ar) | 0.93% | ~1.3% | ~ 0.4 مجم/لتر |
| ثاني أكسيد الكربون (CO₂) | ~0.04% | ~1.5-6% (يختلف على نطاق واسع) | ~0.5 ملغم/لتر (يمكن أن يكون أعلى بكثير في بعض المياه) |
روابط
50 روابط لصفحات أخرى: الأكسجين المذاب
تقوم شركة "أكنتي" بأنشطة التصنيع والتسويق والبيع لأجهزة الفقاعات بتكنولوجيا النانو، ومكثفات الأكسجين الصناعية
تقنية فقاعات النانو من أكنتي، معلومات حول العلم وراء هذه التقنية.
مولدات أكنتي لفقاعات النانو وتعزيز النمو وتحسين التنظيف والتطهير. مستويات عالية من الأكسجين المذاب.
أكنيتي هي شركة تصنيع معدات فقاعات النانو ومُكثفات الأكسجين الصناعية. تختص شركة أكنيتي في بيع وتسويق تكنولوجيا فقاعات النانو. تعد تكنولوجيا فقاعات النانو الثورة التالية في المياه والسوائل لتغيير خصائصها عن طريق إذابة الغازات لتعزيز العمليات البيولوجية والابتكار في التنظيف والتعقيم في مجموعة واسعة من التطبيقات.
أصول وموارد العلامة التجارية أكنيتي، أسلوب الشركة الداخلية.
شروط الخدمة وسياسة الخصوصية الخاصة بشركة أكنتي للفقاعات بتكنولوجيا النانو & ومزوِّد مُكثِف الأكسجين، أوساكا اليابان
مقالات مدونة وأخبار حول الفقاعات النانوية وتكنولوجيا الفقاعات متناهية الصغر ومكثفات الأكسجين.
الفقاعات في كل مكان من حولنا ، في أطعمتنا ، والبيرة ، ومشروبات البوب ، والخبز والجبن ، ولكن أيضًا في أحجار منزلنا. الفقاعات عبارة عن تجاويف مملوءة بالغاز في الماء ، وعمر الفقاعة قصير على الأكثر لبضع دقائق ، فقط الفقاعات فائقة الدقة تكون ثابتة لفترات أطول مثل الأشهر ، وهذا يجعلها مميزة للغاية ويمكننا من تغيير خصائص ماء.
DO levels controller 0-40 ppm
اكتشف جهاز GaLF فائق الدقة GaLF، وهو مولد متطور يوفر أعلى تركيز للفقاعات النانوية في صناعة الفقاعات النانوية. مصمم للباحثين والجامعات والمختبرات، وهو مثالي للأبحاث الأساسية وتطوير المنتجات. وبفضل أدوات التحكم PLC المتقدمة وخيارات الغاز المرنة، تضمن هذه الوحدة المدمجة والقوية أعلى أداء وسهولة التشغيل. اقرأ المزيد لمعرفة كيف يمكن للفقاعات النانوية تعزيز التنظيف ونمو النباتات وصحة الأسماك.
يُعد جهاز miniGaLF نموذجًا بدائيًا من أجهزة GaLF وهو مصمم للشركات والجامعات ومعاهد البحوث والأفراد الذين يرغبون في التعرف على تقنية الفقاعات الدقيقة جدًا.
حلول GaLF المخصصة المضمنة في تطبيقاتك ، هذا نموذج من الفولاذ المقاوم للصدأ. مناسب للهيدروجين والبنزين.
تخيل أن أرضية المطبخ أو الحمام يتم تنظيفها بواسطة فقاعات صغيرة جدًا. يبدو رائعًا، فقد بدأ عصر تكنولوجي جديد.
إن miniGALF هو نموذج GaLF مبتدئ IDEC المصمم للتجارب الأولى مع تقنية فقاعات النانو. يتيح miniGaLF-Plus تركيزًا أعلى من فقاعات النانو عن طريق ممرات متعددة للمياه عبر miniGaLF.
فيلم فقاعات النانو، فيلم باستخدام Malvern NanoSight. استنادًا إلى هذا الفيلم، يقوم سوفتوير NanoSight الداخلي بحساب عدد الفقاعات لحساب تركيز عدد الفقاعات وحجم الفقاعة.
تحسين جودة المياه باستخدام حلول الأكسجين المذاب وتقنية الفقاعات النانوية من Acniti. تحسين التهوية للزراعة وتربية الأحياء المائية والأبحاث.
تحدث الأشياء العظيمة عندما يتفق العالم ، والفقاعات الدقيقة هي تقنية مبتكرة ، ولكن بدون معايير مناسبة لتطوير الصناعة ، هناك عقبات. منذ عام 2013 ، بدأت ISO في إنشاء معيار دولي للفقاعات الدقيقة المعروفة باسم فقاعات النانو.
صفحة الاتصال علي شركة "أكنتي" إذا كان لديك أي استفسار حول منتجاتنا ، كيفية عملها، فوائدها ، أسعارها، تواصل معنا عبر صفحتنا .
هناك تقنيات مختلفة لتوليد فقاعات النانو (فقاعات متناهية الصغر). تقدم هذه المقالة نظرة عامة على التقنيات الأكثر استخدامًا ، مثل الانحلال المضغوط ، والخلاطات الساكنة ، والتدفق الدوراني.
طريقة جديدة ومبتكرة لنقل الأسماك أثناء النوم جاهزة تقريبًا للذهاب إلى السوق. مزيج من CO2 والفقاعات متناهية الصغر يجعل هذا ممكنًا.
فقاعات النانو هي عبارة عن تجاويف مملوءة بالغاز في الماء. منطقة التلامس بين الفقاعات في الماء المليء بالفقاعات الصغيرة أكبر بكثير من المياه المليئة بالفقاعات الأكبر. يكون ضغط الغاز داخل الفقاعة الصغيرة أعلى منه في الفقاعة الكبيرة ، وبالتالي يكون التوتر السطحي للفقاعة الصغيرة أعلى أيضًا.
فلاتر التنظيف الذاتي تمنع انسداد المضخات وتجعل التهوية أكثر كفاءة. هذه المضخة ممتازة أيضًا كفلاتر لنزح المياه لتصريف الخزانات أو المناطق المغمورة. يشرح هذا الفيديو طريقة العمل الداخلية لفلتر المياه الذكي هذا.
فلاتر المدخل، تجنب سد المضخات وتحافظ على حياة مائية آمنة. الفلاتر قابلة للتركيب على المضخات الغاطسة والعادية.
فيديو عرض مرشح المدخل ذاتي التنظيف. ترشيح فعال وتصميم مبتكر لمياه نظيفة.
الفردي RF100 هو أصغر فلتر مدخل (مصفاة) ذاتي التنظيف. يصفي حتى 30 لترًا / الدقيقة وتم تصميمه لحماية المضخات والمعدات الأخرى. هذا الفلتر القوي والموثوق به سهل التركيب. فهو مضغوط وخفيف الوزن ولديه وصلات بسيطة. رائع للاستخدامات الزراعية والصناعية الخفيفة حيث يوجد حاجة إلى تصفية المياه. تستخدم المصفاة 20 - 25٪ من طاقة المضخة لدفع الدوران الخلفي الداخلي للغسيل، وتنظيف الشبكة بأكملها كل ½ ثانية. يتم الحفاظ على الرواسب والحياة البرية والصلبة المعلقة بعيدًا عن شبكة المصفاة.
RF200 هو فلتر ذاتي التنظيف للتوصيل بخرطوم الشفط للمضخات المثبتة على السطح. يمكن تزويدها بشبكة فلتر نايلون متينة أو شبكة من الفولاذ المقاوم للصدأ متكلس ويمكن ترشيحها من 60 إلى 315 ميكرون. تصل سعة فلاتر RF200 إلى 220 لترًا في الدقيقة. تحتوي جميع الفلاتر على دوار تنظيف داخلي ، وهو يعمل بالماء عن طريق إخراج "T" من خرج المضخة لتزويد الدوارات الداخلية للفلتر. يقوم هذا الإمداد بغسل الشاشة الشبكية بشكل عكسي بشكل مستمر بحيث يدفع بعيداً أي شيء قد يسبب انسداد الشبكة. يمكن أن تعمل هذه الفلاتر في المياه المتسخة جدًا دون سد ، مما يقلل من الحاجة إلى خزانات الاستقرار ويقلل من الصيانة إلى الحد الأدنى.
يعد غسل الأسطح والغسيل من المجالات الواعدة التي يمكن أن تحدث فقاعات النانو فرقًا في التطبيق. إن تقليل كمية المنظفات له تأثير إيجابي عن طريق تقليل التلوث. وغسل الملابس بدون منظفات من شأنه أن يفيد البيئة بشكل كبير. يمكن لفقاعات النانو أن تخفض التوتر السطحي للماء ، والكميات الكبيرة من جزيئات الأكسجين في الفقاعات تشحن الماء سالبًا.
يوفر فلتر ومصفاة الشفط الذاتي RF600 قوة صناعية وتصفية ذات صيانة منخفضة للمضخات المثبتة على السطح. وهي عبارة عن فلاتر قوية من الفولاذ المقاوم للصدأ التي تحمي المضخات والمعدات المتصلة الأخرى من الانسداد عند ضخ المياه القذرة. توفر آلية التنظيف الحائزة على جائزة في الفلاتر غسلًا مستمرًا يحافظ على شبكة الفلتر خالية من الأعشاب والحطام والمواد الصلبة الأخرى المعلقة. يمكن لل RF600 التعامل مع 90 متر مكعب إلى 275 متر مكعب في الساعة.
تقبل أكنيتي المدفوعات البنكية و TransferWise و PayPal والعديد من بطاقات الائتمان الرئيسية فيزا و ماستركارد و American Express و Discover و JCB و Diner's Club و EnRoute تقبل أكنيتي المدفوعات بالعملات التالية: اليورو ، والين الياباني ، والدولار الأمريكي ، والجنيه الإسترليني ، والدولار الأسترالي ، والدولار النيوزيلندي ، والدولار السنغافوري
هل أنت مهتم بمعرفة سبب تسمية فقاعات النانو رسميًا بالفقاعات فائقة الدقة؟ في هذه المقالة نوضح الأسباب التي دفعت اللجنة الفنية ISO قررت استخدام الاسم الرسمي فقاعات متناهية الصغر بدلاً من فقاعات النانو.
خس مانوا هو نوع من أنواع الخس المعرضة للإصابة بحروق الأطراف. حرق الأطراف هو تجفيف وموت أنسجة الأوراق على طول حواف الورقة. خلال اختبار في مزارع في هاواي تبين أنه من خلال زيادة مستويات الأكسجين المذاب وإضافة فقاعات متناهية الصغر يتم تقليل حروق الأطراف وتتحسن الجودة والإنتاج.
البروبيوتيك الكائنات الحية الدقيقة الحية التي تعزز الفوائد الصحية للزراعة وتربية الأحياء المائية ومعالجة المياه
تتنافس بروبيوتيك المقدمة من أكنيتي الزراعية مع الميكروبات الضارة. تساعد الأنواع المختلفة من البكتيريا الموجودة في صورة أقراص على إزالة مصدر الطعام للبكتيريا الضارة. يجب حل أقراص الفوار في الماء.
غالبًا ما يكون تكاثر الطحالب سببًا لبدء معالجة الأحواض. يمكن أن يؤدي استخدام البروبيوتيك مع الفقاعات فائقة الدقة إلى زيادة كفاءة عملية معالجة مياه الصرف الصحي. توفر أكنيتي البروبيوتيك على شكل أقراص بطريقة سهلة وآمنة لجرعة البكتيريا المفيدة وتطبيقها.
فوائد تربية بروبوتيك الأحياء المائية
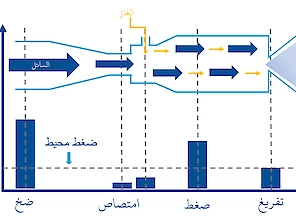
مُكثّف الأوكسجين أوكسيتي الصناعي بمكونات عالية الجودة من اليابان وأمريكا ، مبني في غلاف ألومنيوم متين التصميم. يستخدم المكثف تقنية امتصاص تأرجح الضغط (PSA) وينتج 8 لتر من الأوكسجين في الدقيقة. توفر هذه الوحدة راحة البال وساعات عديدة من إنتاج الأكسجين المستقر.
miniGaLF هو نموذج GaLF لمستوى المبتدئ الخاص بشركة أكنتي وهو مصمم للشركات والجامعات ومعاهد البحوث والأفراد الذين يرغبون في التعرف على تقنية الفقاعات فائقة الدقة. في هذه المدونة ، يتم عرض فيلم عن التوصيلات والأداء لإنشاء فقاعة متناهية الصغر (فقاعات نانو) ذات نسبة عالية من الأكسجين المذاب.
فقاعات النانو مفيدة في تسريع عملية التمثيل الغذائي للكائنات الحية، لكن الآلية ليست مفهومة جيدًا بعد. في دراسة، قاموا بالتحقيق في إنتاج أنواع الأكسجين التفاعلية (ROS) بواسطة فقاعات النانو وتأثيرها على إنبات البذور. كانت نتيجة الدراسة أن البذور الموجودة في ماء فقاعات النانو لها معدل إنبات أعلى من كل تلك المغمورة في المحاليل التقليدية الأخرى المستخدمة.
إعداد مضخة Tsurumi 50TM.275 لخلاط فقاعات النانو توربيتي الغاطس للمياه المالحة.
إعداد مضخة معالجة المياه Tsurumi 50 PN2.75 لخلاط فقاعات النانو توربيتي 737 الغاطس.
تأكد من الأداء الأمثل لمولد الفقاعات متناهية الصغر الخاص بك مع جهاز استشعار الفقاعات النانوية المتناهية الصغر ALT-9F17 - وهو مستشعر متطور للفقاعات النانوية يوفر مراقبة دقيقة وفي الوقت الحقيقي باستخدام طريقة الليزر المتناثرة. اكتشف كيف يساعدك هذا الحل المدمج وسهل الاستخدام على التحكم في جودة المياه وتقليل التكاليف وتعزيز الكفاءة التشغيلية. استكشف مواصفاته الرئيسية وميزاته وفوائد تطبيقه لإطلاق العنان لإدارة المياه بشكل فائق.
خيار المضخة لـ miniGaLF و Lowara PM21 ، تعد المضخة جزءًا من خيار miniGaLF Plus ، استخدم المضخة لإنشاء تركيز أعلى من فقاعات النانو
خيار المضخة لـ miniGaLF و Ebara PRA 0.50 ، تعد المضخة جزءًا من خيار miniGaLF Plus ، استخدم المضخة لإنشاء تركيز أعلى من فقاعات النانو
صمام فحص سواغيلوك لminiGaLF لإعادة تدوير الماء في الخزان وإنتاج تركيز عالي من الفقاعات الدقيقة جدًا.
خيار مضخة أيبارا لخلاط فقاعات النانو الأوزون توربيتي 3LM4 40-160 / 0.55
صمام فحص الغاز الأكسجين المطلوب لجميع خلاطات فقاعات النانو لتجنب دخول الماء إلى مُكثّف الأكسجين
اكتشف مضخات العينات عالية الأداء من Acniti، المصممة بخبرة للاستخدام مع مستشعر الفقاعات النانوية ALT ومستشعرات تركيز الماء بالأوزون. تضمن هذه المضخات المصممة بمتغيرات قوية مقاومة للتآكل وقياسية تحليل دقيق وموثوق للمياه للمختبرات والتطبيقات الصناعية. كما أن توافقها ومتانتها وتصميمها المبتكر يجعلها ضرورية للقياسات الدقيقة القائمة على المستشعرات في المراقبة البيئية ومراقبة الجودة.
cartridge filter 2~7μm for the ALT 9F17 to remove micro bubbles and particles before analyzing the number of nano-bubbles
عوامة لخلاط فقاعات النانو الغاطس. بهذه الطريقة يسهل وضعها في الماء